- Casa
- "
- Articoli in evidenza
- "
- Stent medicale assorbibile al magnesio di terza generazione-99% assorbimento in 12 mesi
Stent medicale assorbibile al magnesio di terza generazione-99% assorbimento in 12 mesi

Recently, the BIOMAG-I study, the first human clinical evaluation of the third-generation drug-eluting absorbable magnesium stent (DREAMS 3G), showed that more than 99% of the stents had completely degraded to the point of being invisible to the naked eye and no stent mesh-beam appositional malapposition was observed in the 12-month post-PCI OCT review. This result indicates that DREAMS 3G has excellent biocompatibility and degradation properties, effectively avoiding the problem of long-term foreign body reaction of traditional permanent stents.
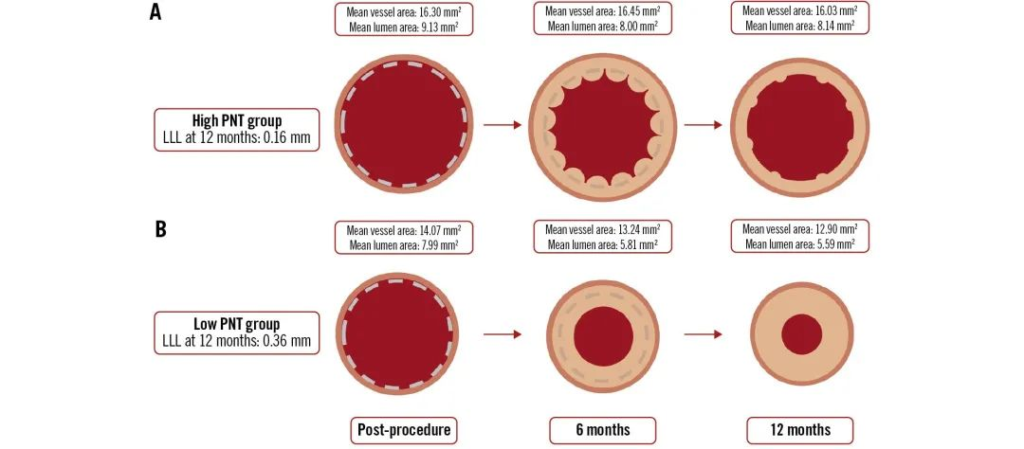
The study systematically assessed key parameters of vessel healing using optical coherence tomography (OCT) and intravascular ultrasound (IVUS) at two time points, 6 and 12 months. The data showed that although the minimum lumen area (MLA) was significantly reduced (from 6.88 mm² to 4.75 mm², p<0.0001) between PCI and 6 months, the MLA was stable by 12 months, suggesting that the vascular structure had matured; meanwhile, the neointima, although evident in 89.3% of the patients at 12 months, was reduced by an average of 47.4%, reflecting the transition of the endothelium from early hyperplasia to a stable, functional structure.

In addition, the DREAMS 3G stent, made from BIOTRONIK’s proprietary BIOmag magnesium alloy and combined with a sirolimus drug coating, not only combines radial support durability while maintaining a 12-month degradation time, but also offers up to 15 size options, significantly improving vascular marker visualisation and implantation safety. The trial showed a low rate of target lesion failure and no stent thrombosis, and late lumen loss (LLL) within the stent also demonstrated significant improvement (mean LLL 0.21 mm, median 0.13 mm).
The BIOMAG-I study provides valuable data on the safety and efficacy of resorbable stents in humans, and lays a solid foundation for further optimisation and clinical dissemination of the technology in the future.